By Dr. Katherine Schrubbe, RDH, BS, MEd, PhD.
Most of us associate the term boot camp with the military. It is described as a basic training that prepares recruits for all elements of service: physical, mental and emotional. It is also labeled as an intense experience that gives service members the basic tools necessary to perform the roles that will be asked of them. The purpose of this training is not to break recruits; in fact, the combination of physical training, field exercises and classroom time makes individuals strong and capable. It’s a tough process, but a rewarding one that many service members value for life.1
In dentistry, where there is a constant pursuit to provide patients with an infection free, safe dental visit, there exists another kind of boot camp. This one isn’t provided by the military, but rather by the dental industry’s Organization for Safety, Asepsis and Prevention (OSAP). This year’s Boot Camp, which took place in January, was themed, Safety Strong. Like military boot camp, it included three intense days of training designed to prepare new and existing dental team members responsible for infection control on the basic tools necessary to perform their roles. Although there was no formal physical training, each day began at 7:30 a.m. sharp and closed around 5 p.m., and included a full schedule of lectures and interactive field exercises, called “boots-on-the-ground” sessions. Interestingly, the completion of OSAP Boot Camp has the same goal as military boot camp: to make dental team members strong and capable of carrying out OSHA standards and CDC best practices for infection control in their dental settings.
This year, I had the honor of being invited to speak at the OSAP Boot Camp conference. One of my assigned topic areas was personal protective equipment (PPE), and there were two objectives to my presentation: First, to define the elements and use of personal protective equipment (PPE) as required by OSHA and CDC recommendations that meet Standard Precautions. Second, to understand the rationale for compliance to standards, regulations, guidelines and best practices. PPE is sometimes an area of compliance that is taken for granted, so this may be a good time for a brief review and reminder on standards related to its importance and use.
Why comply with
 The Occupational Safety and Health Administration (OSHA) is part of the U.S. Department of Labor, and therefore a federal law. OSHA’s mission is to assure safe and healthful working conditions for working men and women by setting and enforcing standards and by providing training, outreach, education and assistance.2 OSHA is there to protect workers from hazards on the job and in healthcare. Dental team members are exposed to a variety of such hazards, such as infectious agents from patients and contaminated equipment, as well as chemicals.
The Occupational Safety and Health Administration (OSHA) is part of the U.S. Department of Labor, and therefore a federal law. OSHA’s mission is to assure safe and healthful working conditions for working men and women by setting and enforcing standards and by providing training, outreach, education and assistance.2 OSHA is there to protect workers from hazards on the job and in healthcare. Dental team members are exposed to a variety of such hazards, such as infectious agents from patients and contaminated equipment, as well as chemicals.
The use of PPE is mandated by OSHA with varying specifications. For dental healthcare workers, the Bloodborne Pathogens standard 1910.1030 clearly states, “when there is occupational exposure, the employer shall provide, at no cost to the employee, appropriate personal protective equipment such as, but not limited to, gloves, gowns, laboratory coats, face shields or masks and eye protection, and mouthpieces, resuscitation bags, pocket masks, or other ventilation devices. Personal protective equipment will be considered appropriate only if it does not permit blood or other potentially infectious materials (OPIM) to pass through to or reach the employee’s work clothes, street clothes, undergarments, skin, eyes, mouth, or other mucous membranes under normal conditions of use and for the duration of time which the protective equipment will be used”.3 In dentistry, PPE act as an efficient barrier to prevent contact with infectious agents and hazardous chemicals.
Another OSHA standard – one which is sometimes overlooked in dental practices, but which must be carried out – is Standard 1910.132, Personal Protective Equipment. It provides additional guidance on employer responsibilities and training related to PPE, stating that employers shall:
- Provide PPE in appropriate sizes.
- Clean, launder and dispose of PPE.
- Repair/replace PPE as needed.
- Ensure PPE is removed before leaving the work area.
- Train employees on proper use.4
Training
Dental employers have a responsibility and obligation to train team members on PPE. During and after training sessions, there should always be time allotted for team members to ask questions and verify expectations. OSHA 1910.132 states that the employer shall provide training about:
- When PPE is necessary.
- What types of PPE are necessary.
- How to properly don, doff, adjust and wear PPE.
- The limitations of PPE.
- The proper care, maintenance, useful life and disposal of the PPE.4
Also, the standard states that the team member shall demonstrate an understanding of the training and the ability to use PPE properly, before being allowed to perform work requiring the use of PPE.4 No dental team member should be permitted to perform duties with occupational exposure without being properly trained. If team members are not utilizing PPE appropriately, constructive corrections should be made immediately. OSHA 1910.132 states, “retrain when the employer has reason to believe that any employee who has already been trained does not have the understanding and skill required.”4 Dental team members should not be permitted to continue to act inappropriately, as it puts them at greater risk for occupational exposure and injury. Training should always be documented in writing to create a permanent record.
Although not a regulatory agency, the Centers for Disease Control and Prevention (CDC) provide key recommendations for PPE that are consistent with OSHA standards as indicated below. Guidelines from the CDC should be strictly followed to reduce the risk of disease transmission to both patients and dental team members.
- Summary of Infection Prevention Practices in Dental Settings: Basic Expectations for Safe Care, 2016.
Protective clothing
Protective clothing, such as clinic gowns, can be reusable or disposable. They are used to protect the wearer from the spread of infection or illness should the wearer come in contact with potentially infectious liquid and solid material; gowns are considered one part of an infection-control strategy.5,6 OSHA requires long sleeves to protect forearms and clinic attire when spray/spatter of blood, saliva or OPIM is anticipated; also based on the information in 1910.1030, the desirable features of a clinic gown are tight cuffs, a high neck and fluid resistance.
Gowns must be changed when visibly soiled, at end of work-shift or whichever comes first, and team members must remove all PPE when leaving treatment area.3 Therefore, gowns are not permitted in non-clinical areas, such as offices, food areas or outside. Often, there are team members who state they take their gown home to launder. This practice is strictly against OSHA; the standard states that laundering is the responsibility of the employer at the office or through contact with a commercial service. “Employees are not permitted to take contaminated gowns or lab coats home for laundering; when removed, they are to be placed in a designated area or container for storage, washing, decontamination or disposal.”3 If there are no laundering facilities on-site and no commercial contract for professional laundering, then disposable gowns should be utilized in the practice.
Face masks
Face masks are a medical device labeled by the FDA and used to protect the mucous membranes of the nose and mouth from contact with sprays/spatter of oral fluids from the patient or items contaminated with patient fluid.5,7 Masks should be worn during any patient care activity, in the lab during grinding or polishing, when using chemical agents and during instrument reprocessing.3,5,7 As a strong reminder, face masks are single-use devices: one mask for each patient. The FDA labels face mask boxes with the universal symbol for a single-use item to ensure there is no question:8
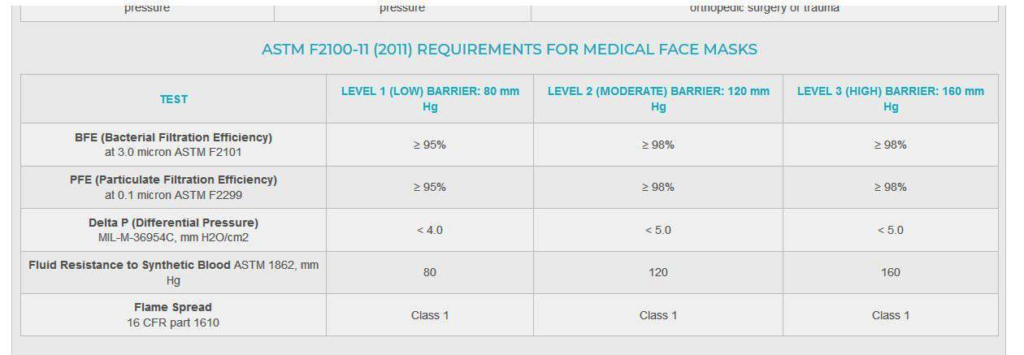
Face masks also are made in a variety of protective levels, and testing is mandatory to determine which of these levels is provided by the American Society of Testing Materials (ASTM).9 The organization develops over 12,500 voluntary consensus standards, but for face masks, the current standard ASTM F2100-11 (2011) specifies the performance requirements for medical face masks with five basic criteria:
- BFE (bacterial filtration efficiency). BFE measures how well the medical face mask filters out bacteria when challenged with a bacteria-containing aerosol. ASTM specifies testing with a droplet size of 3.0 microns containing Aureus. In order to be called a medical/surgical mask, a minimum 95 percent filtration rate is required. Moderate and high-protection masks must have bacterial filtration rates greater than 98 percent.
- PFE (particulate filtration efficiency). PFE measures how well a hospital mask filters sub-micron particles with the expectation that viruses will be filtered in a similar manner. The higher the percentage, the better the mask filtration. Although testing is available using a particle size from 0.1 to 5.0 microns, ASTM F2100-11 specifies that a particle size of 0.1 micron be used.
- Fluid resistance. Fluid resistance reflects the surgical mask’s ability to minimize the amount of fluid that could transfer from the outer layers to the inner layer as the result of a splash or spray. ASTM specifies testing with synthetic blood at pressures of 80 mm, 120 mm or 160 mm Hg to qualify for low, medium or high fluid resistance.
- Delta P (pressure differential). Delta P measures the air flow resistance of the medical mask and is an objective measure of breathability. The Delta P is measured in units of mm H2O/cm2, and the lower the value the more breathable the mask feels. The ASTM standard requires that masks have a Delta P of less than 5.0 for moderate and high barrier masks, while low barrier masks must have a Delta P of less than 4.0.
- Flame spread. As healthcare facilities contain sources of oxygen, heat and fuel, the ASTM F2100-11 standards include testing for flame resistance. Testing dictates that all hospital masks must withstand exposure to a burning flame (within a specified distance) for three seconds.9
In spite of the available guidelines, it can be difficult to determine which face mask will provide the best protection in dental settings. To assist the dental team, ASTM categorizes face mask by level.9
ASTM F2100-11 (2011) REQUIREMENTS FOR MEDICAL FACE MASKS9
Given the possibility of a high amount of spray and spatter of oral fluids, team members should choose a face mask with higher filtration and fluid resistance; sometimes a Level 2 is adequate, however a Level 3 will provide the best protection. For tasks such as patient exams, lab work, taking impressions or operatory processing, a Level 1 mask will suffice. Dental team members should check the information on the mask box to verify its protective level.
Eye protection
Dental team members should wear eye protection with solid side shield to prevents ocular exposure and injury that may occur due to flying objects, spray/spatter of oral fluids and/or aerosols, and hazardous chemicals.3.5 Safety glasses or goggles must also comply with American National Standards Institute (ANSI) Z87.1-2010 to ensure a high level of impact resistancy.10 Every team member must be a part of the infection prevention and safety program and help each other remember to utilize eye protection.
Gloves
Patient care gloves should be worn whenever there is the potential for contact with blood, saliva, mucous membranes, hazardous or infectious wastes or chemical agents.3,5,11,12 Patient care gloves are single-use items; they should always be donned on aseptically clean hands and inspected for tears or holes. They should never be washed or disinfected. In addition, it’s important that dental team members wear the correct size gloves and that they remove them before leaving the treatment area. Both patient care gloves and surgical gloves are regulated by the FDA, however, the non-medical utility gloves are not.12 Utility gloves, which are puncture- and chemical-resistant, should be worn when processing instruments and during housekeeping tasks that involve contact with blood, OPIM or chemical disinfectants.3,5,11 Utility gloves are available both as reusable and disposable. They are highly underutilized, but so very important for the protection of dental team members.
Personal protective equipment is mandated to protect the dental team at the workplace, and not meant to be overwhelming, inconvenient or difficult.
Just like boot camp, this material was intended to provide a review of the basic elements and current information on PPE to prepare new and existing dental team members and those responsible for infection control to perform the roles that will be asked of them. Infection prevention is a team sport and requires team effort in each and every dental setting. It is always a good idea to review the basics, but even a better idea to put them into practice and consistently exercise them.
References
- Today’s Military. Boot camp. Available at https://www.todaysmilitary.com/training/boot-camp. Accessed February 2, 2019.
- U.S. Department of Labor. Occupational Safety and Health Administration. Available at https://www.osha.gov/about.html. Accessed February 2, 2019.
- U.S. Department of Labor. Occupational Safety and Health Administration. Available at https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_id=10051&p_table=STANDARDS. Accessed February 2, 2019.
- U.S. Department of Labor. Occupational Safety and Health Administration.https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_id=9777&p_table=STANDARDS. Accessed February 2, 2019.
- Centers for Disease Control and Prevention. Summary of Infection Prevention Practices in Dental Settings: Basic Expectations for Safe Care. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Oral Health, March 2016.
- U.S. Food and Drug Administration. Medical gowns. Available at https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/GeneralHospitalDevicesandSupplies/PersonalProtectiveEquipment/ucm452775.htm. Accessed February 3, 2019.
- U.S. Food and Drug Administration. Medical devices; Masks and N95 respirators. Available at https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/GeneralHospitalDevicesandSupplies/PersonalProtectiveEquipment/ucm055977.htm. Accessed February 3, 2019.
- U.S. Food and Drug Administration. Medical devices; device labeling. Available at https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/Overview/DeviceLabeling/default.htm. Accessed February 3, 2019.
- American Society for Testing and Materials. ASTM Mask Protection Standards & FAQ. Available at https://www.primed.ca/clinical-resources/astm-mask-protection-standards/. Accessed on February 3, 2019.
- American National Standards Institute. Available at https://www.ansi.org/. Accessed February 3, 2019.
- OSAP. OSHA and CDC Guidelines. Interact training system self-instructional workbook. 2017, section 3.
- U.S. Food and Drug Administration. Medical devices; medical gloves. Available at https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/GeneralHospitalDevicesandSupplies/PersonalProtectiveEquipment/ucm056077.htm. Accessed February 3, 2019.
Editor’s note: Dr. Katherine Schrubbe, RDH, BS, M.Ed, PhD, is an independent compliance consultant with expertise in OSHA, dental infection control, quality assurance and risk management. She is an invited speaker for continuing education and training programs for local and national dental organizations, schools of dentistry and private dental groups. She has held positions in corporate as well as academic dentistry and continues to contribute to the scientific literature. Dr. Schrubbe can be reached at [email protected].