
By Dr. Katherine Schrubbe, RDH, BS, MEd, PhD.
A key process for efficiency, organization and safety
What is the best way to guarantee that your favorite family cake recipe will turn out? There are a few key basics that need to be considered. First, obtain all of the required ingredients: flour, sugar and spices. Next, have the required equipment on hand: mixer, spatulas and pan. Lastly, follow the order of the recipe to ensure a consistent method for baking and serving. In short, be organized and have an efficient, standard process in place.
The same holds true for the management of instruments in a dental practice. Whether the practice is a DSO, a large group or a smaller private practice, the utilization of an instrument management system (IMS) that uses cassettes is key to ensuring organization, efficiency and patient safety. Although organization is not a natural strength for everyone, this management skill is critical in a healthcare environment to promote long-term benefits, such as increased efficiency, effectiveness, productivity and enhanced communication; having no specified systems in place can produce higher stress levels in the workplace.1
Regulatory safety and best practice
According to OSHA’s Bloodborne Pathogens (BBP) Standard (29 CFR 1910.1030), dental practices must identify and use engineering controls. These are devices that isolate or remove the blood-borne pathogens hazard from the workplace.2 Engineering controls are products or devices that have been made or manufactured to help reduce the risk of injury to team members. A cassette is a container that holds instruments for specified procedures and is available in stainless steel, aluminum, plastic or resin material, which can withstand steam, chemical vapor and dry-heat sterilization.3 Cassettes fall under the category of engineering control safety devices, and when incorporated into practice, can reduce the risk of BBP exposure, especially during transporting and reprocessing of dental instruments. In fact, one study showed that 31 percent of sharps injuries occur when instruments are cleaned by hand.4
 Team members assigned to reprocessing instruments in the sterilization area are constantly exposed to potential injuries from contaminated sharps, as well as chemicals; the use of cassettes reduces direct handling of contaminated instruments and keeps the instruments together through the entire process, from chairside to cleaning, sterilizing, storage and presentation to the next patient.3 Cassettes provide an extremely safe avenue because the team member never has to handle or hand-scrub the contaminated instruments. The cassette goes directly into a washer-disinfector or ultrasonic for cleaning.
Team members assigned to reprocessing instruments in the sterilization area are constantly exposed to potential injuries from contaminated sharps, as well as chemicals; the use of cassettes reduces direct handling of contaminated instruments and keeps the instruments together through the entire process, from chairside to cleaning, sterilizing, storage and presentation to the next patient.3 Cassettes provide an extremely safe avenue because the team member never has to handle or hand-scrub the contaminated instruments. The cassette goes directly into a washer-disinfector or ultrasonic for cleaning.
When transporting contaminated sharps from the operatory to the sterilization area, OSHA states that immediately or as soon as possible after use, contaminated reusable sharps should be placed in appropriate containers until properly reprocessed. These containers must be puncture resistant, labeled or color-coded in accordance with this standard and leakproof on the sides and bottom.5 The CDC concurs on this aspect of practice and states team members should minimize handling of loose contaminated instruments during transport to the instrument processing area, as well as use work practice controls (e.g. carry instruments in a covered container) to minimize exposure potential.6 Thus, it is important to note that although cassettes reduce risk, they alone do not meet the criteria of the standard and should be placed on, or into, a secondary transport box, covered tray or container that meets OSHA’s standards for worker safety.
Efficiency
 Being organized improves efficiency. When an IMS with cassettes is used, all of the specified instruments for each procedure are readily available in the cassette. Patients perceive organization and its results. For some, the experience of visiting the dentist can be a source of anxiety, and the professionalism that organization conveys can help reduce this stress. The appearance of the dental operatory can significantly affect a patient’s experience, and an operatory that is clutter-free and organized will be more appealing to patients.1
Being organized improves efficiency. When an IMS with cassettes is used, all of the specified instruments for each procedure are readily available in the cassette. Patients perceive organization and its results. For some, the experience of visiting the dentist can be a source of anxiety, and the professionalism that organization conveys can help reduce this stress. The appearance of the dental operatory can significantly affect a patient’s experience, and an operatory that is clutter-free and organized will be more appealing to patients.1
Efficiency is a win-win for all aspects of the practice. When the operatory is clutter-free and organized, the dentist and assistant are able to function more efficiently as a team. And when the team operates efficiently, a major benefit is increased productivity. The use of an IMS allows the smooth transfer of instruments between team members and permits them to devote more time to patient communication. When the team performs efficiently, they are also more likely to adhere to their scheduled appointment times, thereby helping the front-desk staff plan schedules accurately, without having to explain delays to patients.1
Cassettes also provide efficiency in the clinical setting by reducing the time needed for operatory set-up and clean-up, and facilitating a streamlined process during treatment. If cassettes are labeled by procedure in the storage area, the team member can easily select the type of cassette needed. While working, the cassette can be used on the tray to hold and keep instruments in order, reducing the messy tray problem for providers, as well as patients. As for clean-up, once treatment is complete, the instruments can be returned to their place in the cassette, and the cassette can be closed, locked and transported safely to the sterilization area.1,7
It can’t be overstated: Cassettes keep instruments contained and away from team members, thereby saving time during the reprocessing steps. Practices should always follow CDC guidelines and ensure that all cassettes are wrapped or placed in peel-pouch packages with appropriate chemical indicators prior to sterilization.3,6 The use of cassettes can also maximize counter and storage space in the sterilization area, as they can be neatly stacked and stored more efficiently than trays.
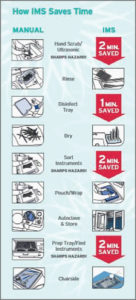
The following graphic illustrates how and where the sterilization steps can be streamlined and time saved when cassettes are utilized.
Graphic courtesy of Hu-Friedy, 2019
Instrument longevity
It is common knowledge that dental instruments are costly for any practice setting, and controlling cost is a high priority. Although it is an investment to implement an IMS, there are long-term benefits that provide numerous advantages, which can have a positive impact on cost containment and instrument life. A few examples include:
- Maintaining inventory effectively. Instruments are already allocated by cassette, so the chance of something being misplaced is reduced, as incomplete set-ups would be apparent. Also, having an organized system for instruments will help maintain the instrument stock and reorders when necessary, rather than at a critical point during a procedure, when the instrument is needed.
- Reducing instrument breakage. Accidental dropping of instruments can occur at any time. However, when instruments are encased in a cassette, they are better protected, substantially reducing breakages. In turn, there will be fewer reorders and less down time due to important instruments breaking at an inopportune time.
- Extending instrument life. Cassettes space items appropriately and generally consist of rails that are constructed of a soft and flexible material. This can prevent the scratching of surfaces and unnecessary bending, and help extend instrument life.
- Improve the sterilization process. When instrument cassettes are loaded correctly and adequately spaced, it allows for complete decontamination (in the case of liquid submersion) and temperature penetration (during thermocycling via an autoclave) to every area of the instruments. The use of an IMS may reduce the possibility of overloading trays or containers during instrument processing and the subsequent loss of sterilization efficacy.8
Just like having the cake turn out perfectly, an Instrument Management System and the use of cassettes provide the practice with the tools needed to ensure organization, efficiency and safety. Large group practices and DSOs tend to be very busy, with definitive metrics and goals and little time for inefficiency. When an IMS is in place, team members can perform their duties without hesitation, indecision or question, knowing that the practice is committed to team safety and efficiency. And the enhanced organization is integral to growing a practice that has methodical systems of well-orchestrated routines – always ready for the next procedure, the next patient, and the next day.1
References
- Lance K, Reil E, Norsted J. Office organization systems enhance practice efficiency and patient experience. Inside Dental Assisting, Vol 7;5, Sept/Oct 2011. Available at https://www.aegisdentalnetwork.com/ida/2011/10/office-organization-systems. Accessed July 12, 2019.
- U.S. Department of Labor. Occupational Safety and Health Administration. OSHA Bloodborne Pathogens fact sheet. Available at https://www.osha.gov/OshDoc/data_BloodborneFacts/bbfact01.pdf. Accessed July 12, 2019.
- Miller CH, Palenik CJ. Infection Control and Management of Hazardous Materials for the Dental Team. 5th ed. St. Louis: Mosby Elsevier; 2013; 128.
- Younai FS, Murphy DC, Kotelchuck D. Occupational exposures to blood in a dental teaching environment: results of a ten-year surveillance study. Journal of Dental Education May 2001, 65 (5) 436-448. Available at http://www.jdentaled.org/content/65/5/436. Accessed July 12, 2019.
- U.S. Department of Labor. Occupational Safety and Health Administration. OSHA Bloodborne Pathogens Standard. Available at https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_id=10051&p_table=STANDARDS. Accessed July 12, 2019.
- Centers for Disease Control and Prevention. Guidelines for infection control in dental health-care settings – 2003. MMWR Recomm Rep 2003;52(RR-17):1-61. Available at https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1030. Accessed July 12, 2019.
- Govoni M. Instrument management systems. RDH; April 1, 2012. Available at https://www.rdhmag.com/infection-control/sterilization/article/16405818/instrument-management-systems. Accessed July 15, 2019.
- Dentalytec. Dental sterilization cassettes; a case study of the pros and cons. December 21, 2016. Available at https://www.dentalytec.com/en/dental-sterilization-cassettes-case-study-pros-cons/. Accessed July 15, 2019.
Editor’s note: Dr. Katherine Schrubbe, RDH, BS, M.Ed, PhD, is an independent compliance consultant with expertise in OSHA, dental infection control, quality assurance and risk management. She is an invited speaker for continuing education and training programs for local and national dental organizations, schools of dentistry and private dental groups. She has held positions in corporate as well as academic dentistry and continues to contribute to the scientific literature. Dr. Schrubbe can be reached at [email protected].

